L2S2 and TTP announce CoolMate joint technology collaboration
TTP and L2S2 have jointly accelerated the development of CoolMate, an engagement solution that enables patients to self-manage their sensitive biologics at home, bringing them freedom and convenience in a safe and controlled manner.
Patients often struggle with self-administering biologic medicines due to simple misunderstanding, forgetfulness, disorganization, mistrust, too many medications, worry, and even depression. Digital interventions and support tools which aim to address these factors have had limited effectiveness to date due to their inability to adapt to user variability whilst also rapidly offering scale.
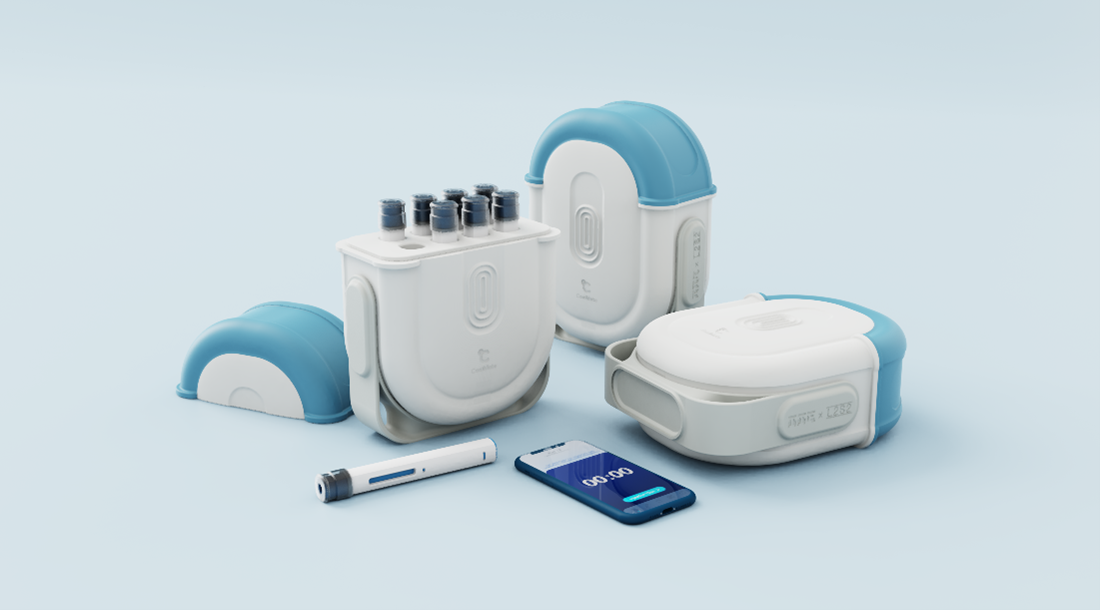
CoolMate is capable of:
- Generating Patient Reported Outcomes (PROs) and engagement data.
- Patient guidance, education, and reassurance (e.g., push notifications).
- Rapid customizability and scalability.
- Medication batch traceability (NFC/RFID).
- Electronic Medical Records (EMR) system and workflow integration, streamlining clinical adoption.
- Medication stock forecasting and responding to unexpected patient-related events.
- Inclusion in clinical trials or for rapid real-world R&D testing by Contract Research Organizations (CROs) and pharmaceutical companies.
Located in Cambridge, UK, TTP plc has been operational since 1987. They are currently an innovation leader in the end-to-end development of connected medical devices and patient-centric design. With strong partnerships across the Pharmaceutical and MedTech sectors, they operate as an independent technology company where scientists, engineers, and designers collaborate to invent and develop new products and technologies.
Contact TTP: digitalhealth@ttp.com
Let's realise your ideas

© 2023 L2S2 Ltd. All Rights Reserved
Privacy Policy | GDPR | ISO 13485:2016 | ISO 27001:2013 | Cyber Essentials | NHS DSP Toolkit | OWASP | HSCN | Mobile Apps

